Lupine publishers | Hypertriglyceridemia and Remodeling of the Left
Ventricle in Patients with Chronic Kidney Disease
Lupine publishers| Journal of Urology & Nephrology Studies

Abstract
Aim: The aim of the study was to study the clinical and
functional features of renal dysfunction in conditions of
hypertriglyceridemia
and remodeling of the left ventricle.
Materials and Methods: 176 patients with chronic kidney disease (CKD) were examined, 111 of them with hypertriglyceridemia
(HTG) in association with left ventricular hypertrophy (LVH) 1st group and 65 patients with LVH in the absence of HTG, matched
by gender and age. Along with general clinical studies, all patients were assessed for lipid spectrum parameters, measurement
of cystatin C in blood plasma, and indicators of arterial stiffness (augmentation index and stiffness) and echocardiography were
analyzed.
Results: In the 2nd group (HTG + LVH), the number
of patients with type 2 diabetes mellitus (DM), a combination of
hypertension,
coronary heart disease and gout were significantly higher (p<0.05).
Persons with chronic obstructive pulmonary disease, chronic
pyelonephritis and cerebrovascular diseases were significantly more
common in the 1st group (p <0.05). In the 2nd group, the value
of BMI [(30.4 ± 5.2 kg/m2 against 28.1±5.7 kg /m2;
p = 0.013), the level of systolic blood pressure (BP) (140 ± 19 mm Hg).
v. 134 ± 18
mmHg; p = 0.042), thickness of the posterior wall of the left ventricle
(LV) (0.98 ± 0.18 cm versus 0.90 ± 0.16 cm; p = 0.008), relative
thickness LV walls (0.385 ± 0.107 units versus 0.357 ± 0.060 units;
p=0.032), the number of patients with concentric LV hypertrophy
(29.2; versus 13.5%; p=0.008), total cholesterol content (5.80
(4.87;6.80) mmol/l vs. 4.75 (4.0;5.38) mmol / l; p=0.000); lowdensity
lipoprotein cholesterol (3.56 (3.12;4.63 ) mmol/l versus 3.09
(2.61;3.79) mmol/l; p=0.045) cystatin C (1.25 (1.08;1.70) mg
/ l versus 1.16 (0.99;1.42) mg/l; p=0.026) turned out to be
significantly higher, and the calculated glomerular filtration rate
(eGFR)
significantly lower (57.0 ± 22 ml / min versus 65.0±23 ml/min; p=0.028)]
compared with the 1st group. A positive correlation was
noted between the level of central BP (r=0.264; p=0.003), the augmentation index (r=0.224; p=0.011) and plasma cystatin C (r=
0.486; p=0.000) with the value of the indexed LV myocardium mass (LVMI) in the total sample surveyed. A negative correlation was
recorded between the eGFR and LVMI (r=-0.425; p=0.000).
Conclusion: In the presence of hypertriglyceridemia in patients with left ventricular hypertrophy, cystatin C increases in blood
plasma and GFR decreases with a tendency for augmentation index to increase.
Keywords: Hypertriglyceridemia; Left Ventricular Remodeling; Chronic Kidney Disease; Cystatin C
Introduction
The number of patients with chronic kidney disease (CKD) is
increasing annually, which is becoming an important medical and
social problem of our time [1,2]. Patients with CKD have an increased
risk of developing cardiovascular diseases (CVD), so they should be
examined to identify these complications [3]. Hypertriglyceridemia
(HTG) can realize its effect on the progression of renal damage
through the development of intrarenal atherosclerosis and through
the toxic effect of lipid particles on nephron structures [4]. It was
shown that already in the early stages of CKD, the plasma TG level
rises, the highest TG levels are detected in patients with nephrotic
syndrome and in patients receiving renal replacement therapy
(RRT) [5]. This is associated with a decrease in the activity of
lipoprotein lipase enzymes and triacylglycerol lipase in the liver.
Under conditions of HTG in renal dysfunction, the production
of proinflammatory cytokines increases and proteinuria is
aggravated, which contributes to the progression of CKD and an
additional increase in cardiovascular risk [6,3]. A decrease in GFR
induces the development of left ventricular hypertrophy (LVH), the
frequency and severity of which determine the prognosis for CKD
[7]. Structural and functional changes of the heart in CKD primarily,
hypertrophy and change in the geometry of the LV, on the one hand,
are considered as a complication, and on the other, as a factor in
the progression of renal dysfunction [8-10]. The combination of
HTG and LVH in the general population of people has been studied
sufficiently. However, there are few works where the value of HTG
as an independent risk for renal dysfunction and CVD would be
considered. This dictates the need to improve the early diagnosis
of heart and kidney damage in HTG. In this regard, the study of LV
restructuring and the functional state of the kidneys among persons
with HTG is of considerable interest.
Materials and Methods
The work was performed in the clinical departments of faculty
therapy of the KSMA n.a.I.K. Akhunbaeva and therapy №2 KRSU
n. a. B.N. Yeltsin, Bishkek. The study included 176 patients over
the age 18 with an established diagnosis of CKD. The midddle age
of the examined individuals was 52.9±13.3 years. Diagnosis of
hypertriglyceridemia [11], left ventricular hypertrophy [12] and
CKD [13] were carried out in accordance with existing standards and
recommendations. The study was approved by the Ethics Committee
of the KSMA n.a. I.K. Akhunbaeva. Nosological characteristics of the
patients included in the study are presented in Table 1. The study
design is a momentary case-control study. The criteria for inclusion
in the study was the presence of echocardiographic (EchoCG) signs
of LVH and CKD. The study did not include individuals with endstage
CKD who are on renal replacement therapy (RRT), or patients
with valvular heart disease. All the examined individuals were
divided into two groups depending on the TG content of the blood
plasma. Group 1 (n = 111) included patients with a plasma TG level
< 1.7 mmol/l, the 2nd group (n=65) with plasma TG ≥ 1.7 mmol/l,
i.e. with the presence of GTG. Physical examination included an
assessment of the general condition, a clinical measurement of
blood pressure (BP) in both hands in a sitting position using the
Korotkov method, a was determined body mass index (BMI), was
calculated and the number of heartbeats (HR). BMI was calculated
using the formula: BMI = body weight (kg)/height2 (m2).
Table 1: Clinical and anamnestic characteristics of research patient.
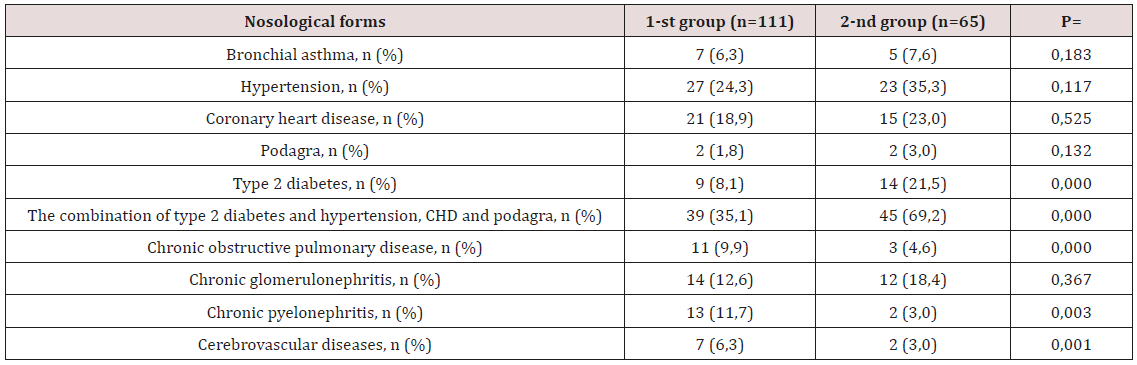
Note: CHD - coronary heart disease; Type 2 diabetes; n- is the number of patients; p-credibility.
The laboratory study included an assessment parameterof lipid
spectrum s (total cholesterol (cholesterol), low-density lipoprotein
cholesterol (LDL cholesterol), high-density lipoprotein cholesterol
(HDL cholesterol) and TG), uric acid, calcium and fibrinogen
in blood. Additionally, all patients were determined by the
concentration of cystatin C in plasma by the immunoturbidimetric
method. The severity of renal dysfunction was determined on the
basis of the GFR, calculated according to the F.J. Hoek formula:
GFR = 80.35 / Cys-4.32 [14]. The instrumental part of the work
consisted of studying the parameters of arterial stiffness and
augmentation (Alp, augmentation index) using the Angioscan-01
device (AngioScan Electronics LLC, Russia) [15]. Transthoracic
EchoCG with simultaneous tissue myocardial dopplerography
was performed on a Vivid Q ultrasound scanner (USA, 2014)
according to the standard technique. The thickness of the walls, the
dimensions of the left ventricular cavity (LV), the diameter of the exit
aorta (cm) and left atrium (cm) were estimated from parasternal
access along the long axis of the LV. Measured the thickness of the
interventricular septum (IVS, cm) and the posterior wall of the left
ventricle (LV, cm) in diastole, the final diastolic (FDM, cm) and the
final systolic dimensions (FDM, cm) of LV were determined.
LV systolic function was estimated by its ejection fraction (EF,
%), which was calculated using the formula L.E. Teichholtz (1976)
in the absence of zones of hypo- and akinesis [16]. In order to assess
the diastolic function of the left ventricle in the mode of pulsed
Doppler sonography, the transmitral diastolic flow rate (E / A) and
the time of blood flow slowing down of the early diastolic filling of
the left ventricle (DT) were measured. The LV myocardium mass
(MLMH) was calculated by the formula R.B. Devereux et al [17].
(1986): MLM (g) = 0.8 - {1.04 - (KDR + MZhP + ZSLZH) 3 - KDR3}
+0.6 [17]. The LV myocardial mass index (LVMH) was defined as the
ratio of LVML to body surface area. The criteria for LVH and types
of LV myocardial remodeling were determined in accordance with
the recommendations of ESC from 2013 [12]. For the assessment
of LVHL, the LVMI was calculated, the upper value of which was 95
g/m2 for women and 115 g/m2 for men. The relative wall thickness
(RWT) of the LV was calculated for each patient as (IVM + LVLS)/
LV CRD. For an increase in RWT, was taken a value of more than
0.42 [12]. The criteria of concentric and eccentric variants of LVHL
were considered to be LVMI values greater than normal, RWT>
0.42 and LVMI higher than normal, but RWT <0.42, respectively.
Statistical analysis of the data was carried out using the software
package “Statistica 10.0”. All quantitative variables are presented
as mean ± standard deviation, medians and quartiles [Me (Q25;
Q75)]. Significance of differences between groups was assessed
using t-Student test (for variables with a normal distribution)
and the Mann-Whitney test (for variables with a non-parametric
distribution) [18]. Correlation analysis was carried out by the
Pearson criterion - for variables with a normal distribution and
the Spearman coefficient (for variables with a non-parametric
distribution). For all types of analysis, p <0.05 were considered
statistically significant.
Results of Research
The distribution of patients with LVH without HTG and with
HTG is presented in Table 1. Among the examined groups with
HTG, the proportion of patients suffering from type 2 diabetes
mellitus (DM) and its combination with hypertension, coronary
disease, and gout was significantly higher in the 2nd group
compared with the 1st group. In contrast, individuals with chronic
obstructive pulmonary disease (COPD), chronic pyelonephritis and
cerebrovascular diseases (CVD) were significantly more common
in the 1st group. When assessing the general characteristics of the
studied groups, it was noted that the initial patients were similar
in age and sex (Table 2). In the 2nd group, i.e. in patients with HTG,
the value of BMI was significantly higher (30.4±5.2 kg/m2 against
28.1±5.7 kg/m2; p=0.013) compared with the 1st group. Analysis of
hemodynamic parameters showed a higher level of systolic blood
pressure (140±19 mm Hg versus 134±18 mm Hg; p=0.042) in the
group of individuals with HTG compared with the 1st group. It
should be noted that there were no intergroup differences in heart
rate, diastolic and central blood pressure, augmentation index and
stiffness (Table 2). According to EchoCG, the diameter of the output
aorta, the end systolic and diastolic size of the LV, the longitudinal
size of the left atrium, the LV ejection fraction and LVMLI did not
differ significantly. There was a tendency to increase the thickness
of the IUP in the group of persons with HTG. At the same time, in
this group there was a statistically significant increase in the SLWL
thickness (Table 2). All examined individuals in both groups had
similar LVML, E/A and DT LV values. Significant differences between
patients of the two compared groups were determined in terms of
LV RWT. As can be seen from Table 2, the RWT of LV patients of the
2nd group significantly exceeded the RWT of the LV patients of the
1st group (p=0.032).
Table 2: Clinical - laboratory parameters of the examined
patients with CKD.
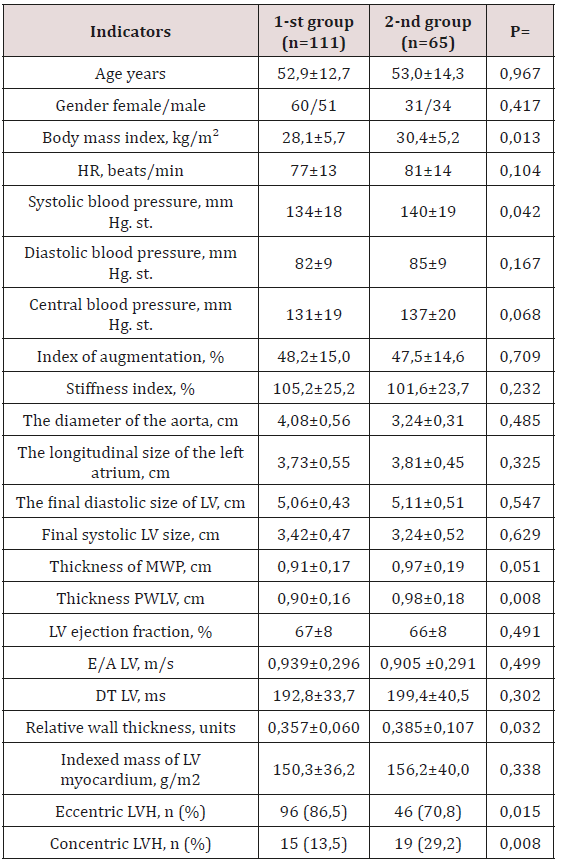
Note: n- is the number of patients; p - reliability; HR - heart rate;
BP - blood pressure; LV - left ventricle; LVH - left ventricular
hypertrophy; DT is the flow deceleration time.
Analysis of the frequency of occurrence of types of LV
restructuring of the two compared groups showed a significant
difference in values (p<0.05). Thus, the proportion of patients
with a concentric type of LVH was significantly higher among
patients with HTG (29.2% vs. 13.5%; p=0.008), and the eccentric
type significantly prevailed in the 1st group (86.5% vs. 70.8
%; p=0.015). At the next stage of the study, were analyzed the
biochemical parameters of the examined groups, the results of
the analysis are presented in Table 3. As was to be expected, in
the group of individuals with HTG, the median total cholesterol,
TG and LDL cholesterol was significantly higher (p <0.05). The
calcium content and the number of patients with an increase in
CRP in the blood plasma were equivalent in both groups. A clinically
significant increase in plasma uric acid levels was recorded in the
2nd group. At the same time, in the same group of patients, there
was a significant increase in plasma cystatin C content (p<0.05)
and a decrease in calculated GFR (p<0.05). Evaluation of the
relationship between the value of LVMI and clinical and laboratory
parameters was carried out first in the general and in each group
separately (Table 4). The presence of a reliable positive correlation
relationship was obtained between the level of central BP (r=0.264;
p=0.003), the augmentation index (r=0.224; p=0.011) and the
concentration of cystatin C in the blood plasma (r=0.486; p=0.000)
total sample. Feedback was observed between eGFR and LVMI (r=-
0.425; p=0.000). Similar correlation shifts with the exception of the
augmentation index also occurred in the 1st group (Table 4). In the
2nd group, a direct close direct connection was found between the
content of cystatin C in blood plasma, the level of central BP with
the value of LVMI.
Table 3: Parameters of biochemical analysis of blood of the examined groups.
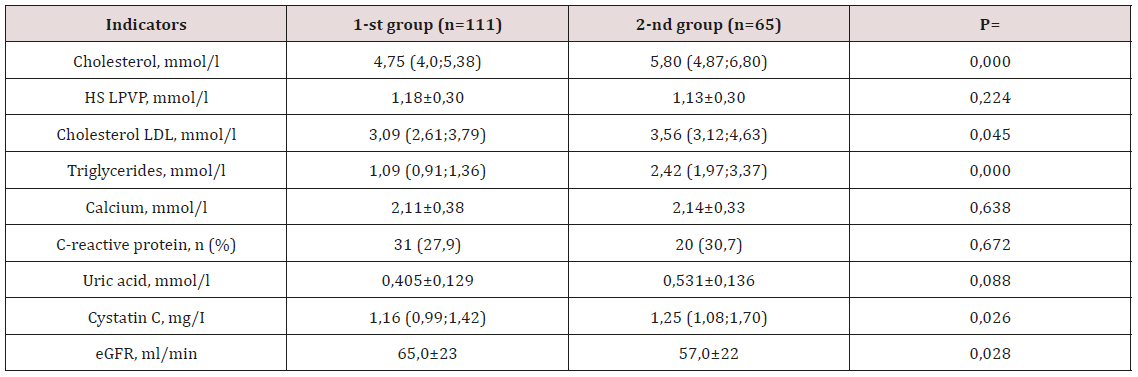
Note: eGFR - estimated glomerular filtration rate. HDL cholesterol - high density lipoprotein cholesterol; LDL cholesterol - low
density lipoprotein cholesterol; number of patients.
Table 4: Correlation analysis between clinical laboratory parameters and the value of LVMI in two groups.
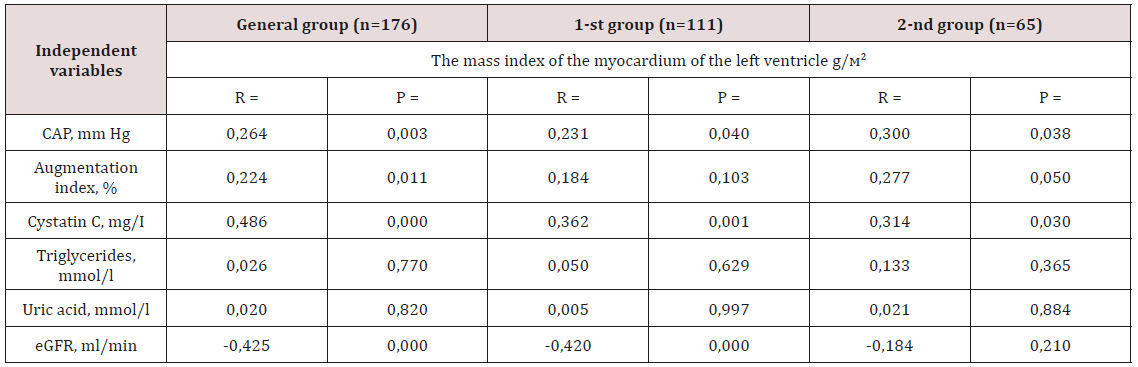
Note: CAP - central arterial pressure; eGFR - estimated glomerular
filtration rate; p - reliability; n is the number of patients; r is the
correlation coefficient.
Discussion
Despite advances in the study of diseases underlying CKD, the
prognosis for this category of patients remains unfavorable. The
concentration of TG-rich lipoproteins increases in the blood plasma
at the early stages of CKD even at normal values of creatinine [19]. It
should be noted that HTG is the most frequent variant of dyslipidemia
in patients with kidney pathology [20,21]. A combination of HTG and an increase in the LV myocardium mass with a change in its
geometry is believed to be associated with a further decrease in
renal function and the risk of mortality, especially in the terminal
stages of CKD [22,23]. It is important to note that HTG in patients of
therapeutic profile has a double meaning. First, HTG is an important
factor in the development and course of multifocal atherosclerosis
and related cardiovascular complications. Secondly, an increase
in the level of TG is directly related to the risk of developing renal
dysfunction [20,21,24]. However, high TG levels are often combined
with low HDL cholesterol levels and high LDL cholesterol levels.
This fact is completely consistent with our results, i.e. in the group
of patients with HTG, there was a statistically significant increase
in the concentration of total cholesterol and LDL cholesterol (Table
3). Some meta-analyzes of TG are identified as an independent
risk factor for CVD [24,25]. Moreover, recent genetic studies have
confirmed the view that elevated TG is a direct cause of CVD
development [26,27]. According to our data, among patients with
HTG, the average eGFR was significantly lower (Table 3). It was
established that a slight decrease in GFR is reflected by changes in
the concentration of cystatin C in the blood [28].
Serum cystatin C levels are considered as a sensitive marker
of the severity of renal dysfunction and the risk of LVH [29]. In
studies A. Shankar et al. (2011), it has been shown that higher
levels of cystatin C in individuals with CKD are associated with
arterial hypertension [30]. In our study, we were also able to
demonstrate a positive correlation between the content of cystatin
C in blood and the value of LVMI in all groups (Table 4). The results
of our work are consistent with data from other studies that have
shown a relationship between elevated levels of cystatin C and
LVH [31,29]. In persons with CKD, LVH is much more common,
and the life expectancy of a patient with renal dysfunction and left
ventricular hypertrophy is significantly reduced [32]. HTG also
contributes to the occurrence of LVH in patients with CKD [20]. The
presence of HTG stimulates the formation of adhesion molecules,
the adherence of leukocytes to the surface of the endothelium,
increases the secretory activity and proliferation of macrophages
in the atherosclerotic plaque, activates the migration and
proliferation of smooth muscle cells. A consequence of these effects
is the development of endothelial dysfunction, increased stiffness
of the vascular wall and myocardium [20,21]. Atherosclerotic
changes in the arterial bed lead to ischemia of cardiomyocytes,
activation of necrosis and apoptosis [33,34]. The decrease in the
number of functioning cardiomyocytes, interstitial remodeling
is accompanied by the development of both systolic and diastolic
myocardial dysfunction [35].
We discovered a direct relationship between the magnitude
of the augmentation index and the presence of LVH in the general
group. A direct relationship was established between the increase
in the augmentation index and the level of systolic blood pressure
[36]. Was shown a close relationship of the blood lipid spectrum
with vascular stiffness indicators [37]. The increase in the stiffness
of large arteries plays an important role in the pathogenesis of
many CVDs and, above all, in the development of hypertension
[38,39]. On the other aspect, an increase in blood pressure
causes changes in the vascular wall, incl. with the development of
arteriosclerosis, which can lead to an increase in arterial stiffness
of the renal arteries [40]. The augmentation index is a surrogate
indicator of arterial stiffness and determines the state of the
vascular bed from the central arteries to the microvasculature
[41]. As a result of an increase in the augmentation index, the load
on the LV increases, which certainly leads to the development of
LVH [42,43]. Concentric hypertrophy is the most unfavorable type
of remodeling, which is associated with the greatest number of
complications [44-46]. The role of LVH and its relationship with
the clinical and laboratory manifestations of CKD at the predialysis
stage continue to be studied. According to the results of our study,
HTG and LVH were reliably accompanied by an increase in systolic
blood pressure, BMI, cystatin C, LDL LDL-C and a decrease in GFR.
The negative impact of these clinical and laboratory parameters on
the LV myocardium was manifested by an increase in the number of
patients with a concentric type of LVH.
Conclusion
Hypertriglyceridemia in patients with left ventricular
hypertrophy is accompanied by an increase in blood cystatin C, a
decrease in GFR, and a tendency for augmentation index to increase.
For More: Lupine Publishers Open Access Journals Please visit our Website:
https://lupinepublishers.us/
https://twitter.com/lupine_online




