Lupine publishers | Journal of Urology & Nephrology Studies
Background: We aim to document if any difference exists for renal functions between metastatic and non-metastatic patients.
Methods: The study population included 12 metastatic and 15 non-metastatic patients. Metastatic renal cancer patients using the TKIs were compared to non- metastatic patients.
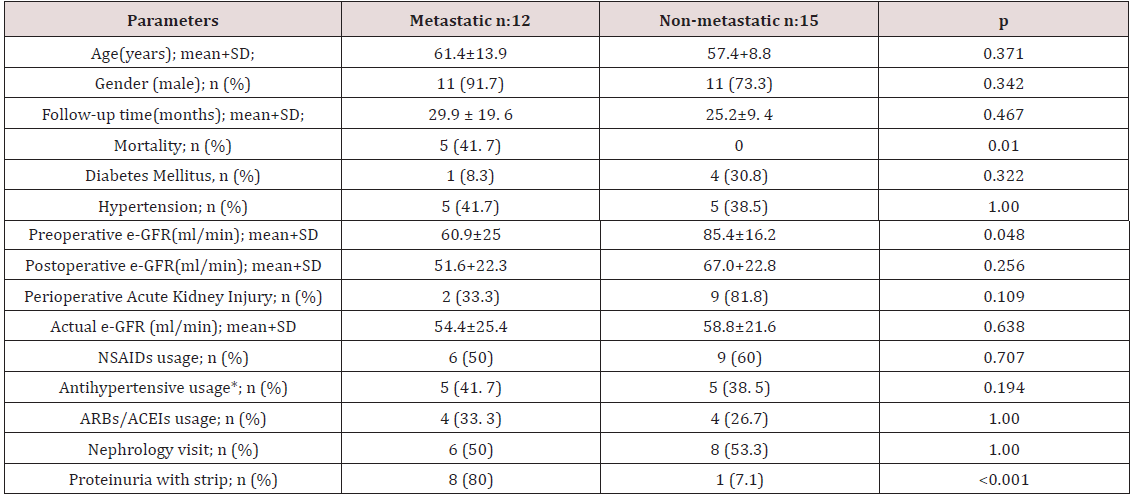
Results:Preoperative estimated glomerular filtration rate (e-GFR) was significantly low in metastatic patients than nonmetastatic patients (p: 0.048). A trend toward increased acute kidney injury during hospital stay in the non-metastatic group was observed, but this fell just short of statistical significance (p: 0.109). Two groups did not differ significantly in terms of postoperative e-GFR (p: 0.256). No statistically significant differences were observed in actual e- GFR between two groups (p: 0.638). No statistically significant differences were found in pre-TKIs and post-TKIs e-GFR values (p: 0.735). Proteinuria was statistically more common in metastatic patients than non-metastatic patients (p<0.001). No statistically significant difference in age, sex, follow-up period, NSAIDs use, antihypertensive and ARBs/ACEIs use were documented between the two groups.
Conclusion: Increased risk for proteinuria was documented in metastatic patients with TKIs use. However, use of the TKIs had no effect on e- GFR. No statistically significant differences were observed in actual e- GFR between two groups.
keywords: Hypertension, Glomerular Filtration Rate, Proteinuria, Tyrosine- Kinase Inhibitors
Abbrevations: RCC: Renal cell carcinoma , TKIs: Tyrosine-Kinase Inhibitors, AKI: Acute Kidney Injury, NSAIDs: Non-Steroidal Anti-Inflammatory Drugs, ARBs: Angiotensin Receptor Blockers, ACEIs: Angiotensin Converting Enzyme Inhibitors, ARBs: Angiotensin Receptor Blockers, CCBs: Calcium Channel Blockers, e- GFR: Estimated Glomerular Filtration Rate
Renal cell carcinoma (RCC) accounts for approximately 3%
of adult malignancies [1]. Treatment options for the RCC include
surgery, radiotherapy and immunotherapy [2]. Besides these
options, molecular-targeted therapies in the form of tyrosinekinase
inhibitors (TKIs) are commonly used. These agents are
well tolerated compared to previously used options (interleukin
2 or interferon-α) and the use of these agents results in longer
progression-free survival and increased response rates [3-5].
Despite the advances achieved with these agents, use of the
TKIs results in side effects such as fatigue, nausea, diarrhea,
gastrointestinal hemorrhage, dysphonia, and palmar-plantar
erythrodysaesthesia [6]. Hypertension and proteinuria are also
usually encountered after the start of these agents [7-9]. In this
study, we compared clinical features between patients with renal
cell carcinoma with and without the TKIs treatment and aim to
document if any difference exists for renal functions between
metastatic and non-metastatic patients during their follow up
period.
The patients diagnosed with the RCC and followed up at
Istanbul Health Sciences University Haydarpaşa Numune Education
and Research Hospital oncology polyclinics between 2009 and
2016 were evaluated. Eligible 12 metastatic patients who received
a nephrectomy and were treated with the TKIs were included in
the metastatic group. Fifteen non-metastatic RCC patients who
only received a nephrectomy formed the non-metastatic group.
Metastatic patients who did not receive a nephrectomy, patients
on dialysis, and patients with insufficient data were excluded.
Patients’ demographics and laboratory data were collected from
the hospital records and via telephone. The clinical, demographic,
and laboratory parameters were compared between the two
groups. The data collected included patient age, sex, follow-up
time, nephrology follow-up, mortality, history of perioperative
acute kidney injury(AKI), use of non-steroidal anti-inflammatory
drugs (NSAIDs), use of antihypertensive medications including
angiotensin converting enzyme inhibitors (ACEIs) and angiotensin
receptor blockers (ARBs), calcium channel blockers(CCBs),
and alpha and beta blockers. The data also included laboratory
parameters such as estimated glomerular filtration rate (e-GFR)
(calculated using the Chronic Kidney Disease Epidemiology
Collaboration (CKD-EPI) equation 2) and proteinuria (assessed
using dipstick and 24-hour urine collection). Creatinine levels
before the operations were used to calculate postoperative e-GFR,
and postoperative creatinine levels taken just before hospital
discharge were used to calculate postoperative e-GFR. Actual e-GFR
was calculated using creatinine levels measured at the last hospital
visit. Perioperative AKI was defined using the acute kidney injury
network classification system. Increase in serum creatinine of 0.3
mg/dl or more within 48 hours or ≥ 1.5 times baseline within 7
days was used in order to define perioperative AKI. Urine volume
was not used as a criterion because data for urine volume were not
available.
The TKIs used as targeted therapy for our patients were sunitinib, pazopanib, and axitinib. Available creatinine measurements before and after the TKIs treatment were used to present the effect of the TKIs on renal function. Statistical analyses were performed using the MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium; http://www. medcalc.org; 2013). The normality of continuous variables was investigated using the Shapiro-Wilk test. Descriptive statistics are presented using the mean and standard deviation for normally distributed variables and median (and minimum–maximum) for the non-normally distributed variables. Student’s t-test was used to compare two normally distributed groups. Non-parametric statistical methods were used for values with skewed distributions. The Mann-Whitney U test was used to compare two non-normally distributed groups. The χ² test was used for categorical variables and expressed as observation counts (and percentages). Statistical significance was accepted when the two-sided p value was lower than 0.05.
The study population consisted of 22 males and 5 female
patients. The mean age of study population was 59.2±11.3
years. Clinical and laboratory parameters of metastatic and nonmetastatic
patients are listed in Table 1. Preoperative e-GFR was
significantly lower in metastatic patients when compared to nonmetastatic
patients (p: 0.048). When two groups were compared,
a trend toward the increased AKI during hospital stay in the nonmetastatic
group was observed, but this fell just short of statistical
significance (p: 0.109). There was no statistically significant
result between two groups when two groups were compared for
postoperative e-GFR (p: 0.256). The statistically nonsignificant
result for postoperative e-GFR between two groups was also
observed for actual e-GFR (p: 0.638) and no statistically significant
differences were found between pre-TKI and post-TKI e-GFR values
(p: 0.735). Proteinuria was more common in metastatic patients
than non-metastatic patients (p<0.001). In metastatic group 8
patients had proteinuria. Four patients out of these 8 patients had
24-hour proteinuria measurements. One patient had nephrotic
range proteinuria and the other three patients had non-nephrotic
range proteinuria. No biopsies were performed on these patients
with proteinuria. Two metastatic patients had negative results for
proteinuria. Data for dipstick proteinuria were unavailable for the
other two patients. Only one patient in the non-metastatic group
had proteinuria and it was at a level of less than 1 gr/day. There was
no statistically significant differences in age, sex, follow-up period,
NSAIDs use, antihypertensive and ARBs/ACEIs use, and follow-up
time between metastatic and non- metastatic patients. Follow-up
times for metastatic and non-metastatic patients were 29.9±19.6
and 25.2±9.4 months; respectively. Three patients died during the
follow-up period and all were in the metastatic group.
e-GFR: Estimated glomerular filtration rate; NSAİDs: Non-steroidal anti-inflammatory drugs; ARBs/ACEIs: angiotension receptor blockers/ angiotension converting enzyme inhibitors; * antihypertensive use rather than ARBs/ACEIs
Nearly all patients taking the TKIs experience a rise in blood
pressure. Systemic vasoconstriction and volume overload are
parts of the mechanisms responsible for systemic hypertension
caused by TKIs [7]. Despite the risk of hypertension occurrence,
the development of hypertension is mostly a good prognostic sign
because it is associated with longer progression free and overall
survival and can be used as a biomarker for tumor responsiveness
[10]. Regarding our study, there was no statistically significant
results between groups for ACEIs/ARBs and other antihypertensive
use. Proteinuria is another important side effect that can be
encountered after the start of targeted agents. In the kidney,
the vascular endothelial growth factor pathway is known to be
responsible for proteinuria after the start of TKIs [11]. In the study
of Baek et al. [12] initiation of sunitinib therapy was related to
proteinuria and aggravation of preexisting proteinuria in 17.6% and
23.1% of patients; respectively [12]. Again in the COMPARZ study,
discontinuation of treatment because of proteinuria was observed
in 3% and 1% the of patients treated with pazopanib and sunitinib;
respectively [13]. In our study, most of the patients treated with
the TKIs had proteinuria. However, only one patient had nephroticrange
proteinuria. We did not perform a renal biopsy on this patient
but important data can be obtained through this procedure. Biopsyproven
acute interstitial nephritis, thrombotic microangiopathy
and acute tubular necrosis have also been encountered after the
TKIs use [14-16]. In our study, there was no statistically significant
difference between pre-TKIs and pre-TKIs e-GFR values. However,
patients can develop renal insufficiency during treatment with
targeted agents. A study by Zhu et al. [17] showed the development
of renal insufficiency in the RCC patients receiving sunitinib
[17]. In our study, despite the statistically significant result for
preoperative e- GFR in favor of non- metastatic patients, there was
no statistically significant results for postoperative e-GFR between
two groups which may be due to the high percentage of patients
with AKI in the non-metastatic group. Despite the tumor burden
and use of the TKIs in metastatic patients, the non-significant result
for postoperative e-GFR between two groups did not change at
last hospital visit. The small sample size and retrospective nature
of the study are important limitations that should be considered.
However, renal side effects encountered after use of the TKIs
were emphasized in this study with comparing metastatic and
non-metastatic RCC patients. In conclusion, increased risk for
proteinuria was documented in metastatic patients with TKIs use.
However, use of the TKIs had no effect on e- GFR. There was also
no statistically significant difference for the actual e-GFR between
the two groups despite the higher preoperative e-GFR observed for
non- metastatic patients.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional approval has been obtained.
Abstract
Methods: The study population included 12 metastatic and 15 non-metastatic patients. Metastatic renal cancer patients using the TKIs were compared to non- metastatic patients.
Results:Preoperative estimated glomerular filtration rate (e-GFR) was significantly low in metastatic patients than nonmetastatic patients (p: 0.048). A trend toward increased acute kidney injury during hospital stay in the non-metastatic group was observed, but this fell just short of statistical significance (p: 0.109). Two groups did not differ significantly in terms of postoperative e-GFR (p: 0.256). No statistically significant differences were observed in actual e- GFR between two groups (p: 0.638). No statistically significant differences were found in pre-TKIs and post-TKIs e-GFR values (p: 0.735). Proteinuria was statistically more common in metastatic patients than non-metastatic patients (p<0.001). No statistically significant difference in age, sex, follow-up period, NSAIDs use, antihypertensive and ARBs/ACEIs use were documented between the two groups.
Conclusion: Increased risk for proteinuria was documented in metastatic patients with TKIs use. However, use of the TKIs had no effect on e- GFR. No statistically significant differences were observed in actual e- GFR between two groups.
keywords: Hypertension, Glomerular Filtration Rate, Proteinuria, Tyrosine- Kinase Inhibitors
Abbrevations: RCC: Renal cell carcinoma , TKIs: Tyrosine-Kinase Inhibitors, AKI: Acute Kidney Injury, NSAIDs: Non-Steroidal Anti-Inflammatory Drugs, ARBs: Angiotensin Receptor Blockers, ACEIs: Angiotensin Converting Enzyme Inhibitors, ARBs: Angiotensin Receptor Blockers, CCBs: Calcium Channel Blockers, e- GFR: Estimated Glomerular Filtration Rate
Introduction
Materials and Methods
The TKIs used as targeted therapy for our patients were sunitinib, pazopanib, and axitinib. Available creatinine measurements before and after the TKIs treatment were used to present the effect of the TKIs on renal function. Statistical analyses were performed using the MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium; http://www. medcalc.org; 2013). The normality of continuous variables was investigated using the Shapiro-Wilk test. Descriptive statistics are presented using the mean and standard deviation for normally distributed variables and median (and minimum–maximum) for the non-normally distributed variables. Student’s t-test was used to compare two normally distributed groups. Non-parametric statistical methods were used for values with skewed distributions. The Mann-Whitney U test was used to compare two non-normally distributed groups. The χ² test was used for categorical variables and expressed as observation counts (and percentages). Statistical significance was accepted when the two-sided p value was lower than 0.05.
Results
e-GFR: Estimated glomerular filtration rate; NSAİDs: Non-steroidal anti-inflammatory drugs; ARBs/ACEIs: angiotension receptor blockers/ angiotension converting enzyme inhibitors; * antihypertensive use rather than ARBs/ACEIs
Discussion
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional approval has been obtained.
To
Know More About Open Access Publishers
Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment